Data, Reporting, and Evaluation
The types of data involved with the National Diabetes Prevention Program (National DPP) lifestyle change program and the processes and protocols by which that data is shared among parties are similar to other data and data-sharing processes used by payers (e.g., state Medicaid agencies, Medicaid managed care organizations (MCOs), and commercial plans) in their normal business operations with each other and with medical providers. These data and other sources can also be used in the evaluation of the National DPP lifestyle change program and associated pilots.
This page includes the following sections:
- Types of Data
- Data Process Flows
- Data Security and Regulatory Compliance
- Data Sharing and Ownership
- Evaluation
Types of Data
Payers, CDC-recognized organizations, and third-party organizations, if used, will need to establish procedures to exchange the following data:
- Medicaid eligibility information
- Program enrollee contact information
- CDC-recognized organization encounter data
- Claims data
- Cost data
CDC-recognized organizations maintain participant data such as attendance, weight, minutes of physical activity, etc., as required by the CDC’s Diabetes Prevention Recognition Program (DPRP), which sets the standards for CDC recognition and serves as a neutral quality assurance function to assure quality and fidelity to scientific evidence. CDC-recognized organizations can consider entering into an umbrella hub arrangement (UHA) to help aggregate DPRP data and streamline business and administrative duties. For more information, visit the Umbrella Hub Arrangements pages of the Coverage Toolkit.
Note: Only entities that elect to become CDC-recognized organizations are required to submit deidentified data to CDC.
Data Process Flows
The following are the key process flows/data exchange frameworks that the relevant parties will need to put in place to facilitate program participation, ensure an appropriate reimbursement framework is established between CDC-recognized organizations and applicable payers, and support program evaluation:
Medicaid Agencies
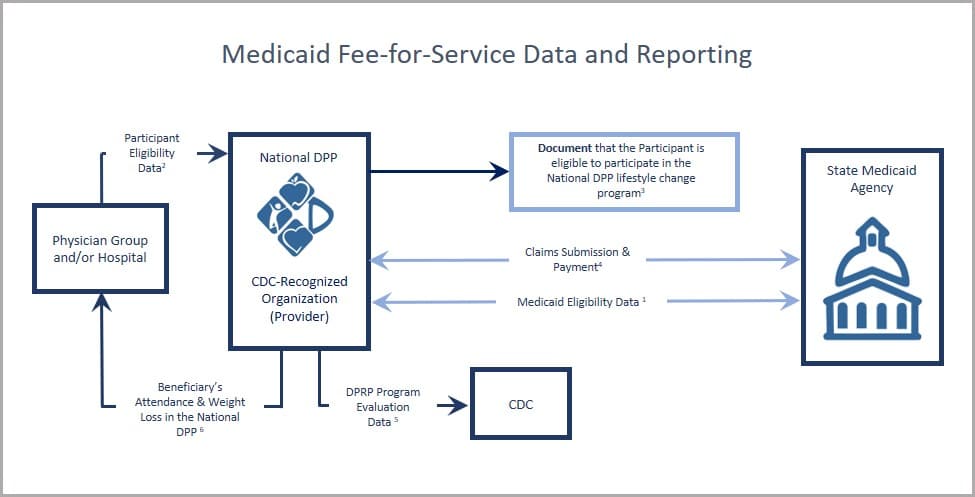
- Beneficiaries are enrolled in Medicaid and eligible for services at the time they are furnished;
- Physician group and hospital may provide electronic health record data to identify enrollees for outreach efforts (for more information, see Screening & Identification);
- To the extent required to be documented by the state, maintain evidence that the participant is eligible to take part in the National DPP lifestyle change program;
- CDC-recognized organization submission of a claim or invoice and encounter data to the state Medicaid agency for reimbursement and evaluation purposes;
- CDC-recognized organization collection of and submission to CDC of required program evaluation data elements for purposes of receiving “pending,” “preliminary,” or “full recognition” designation status. (For more information, see DPRP requirements);
- Experience has shown that provider referrals and sharing National DPP lifestyle change program results with a participant’s primary care provider can increase program enrollment and retention.
Medicaid MCOs
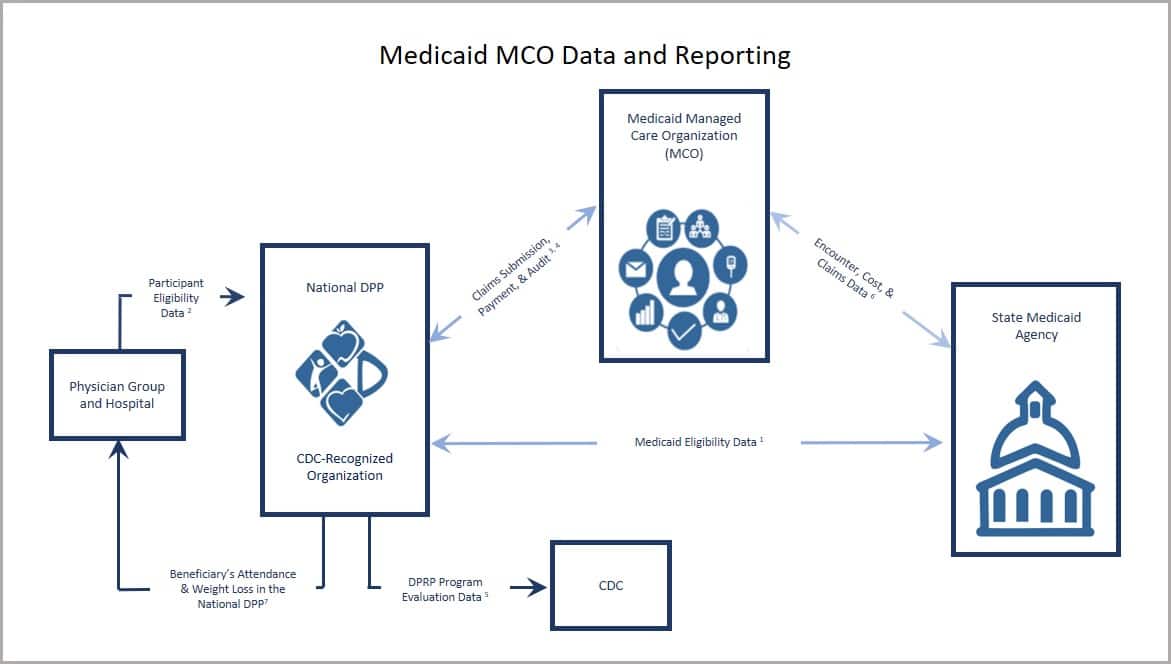
- CDC-recognized organization access Medicaid eligibility data from the state Medicaid agency (e.g., via the eligibility verification system) to confirm that an individual eligible for the National DPP lifestyle change program is enrolled in Medicaid.
- Physician group and hospitals can provide evidence of program eligibility as part of a referral and/or outreach effort to a CDC-recognized organization. Data sharing agreements may be required if lists of potential participants are going to the CDC-recognized organization directly for outreach purposes (for more information, see Program Delivery: Screening & Identification).
- CDC-recognized organization submission of a claim or invoice to the Medicaid MCO (or third party organization, if used) for reimbursement and evaluation purposes.
- CDC-recognized organization or Medicaid MCO submission of a claim or invoice and encounter data as a way to track quality improvements, meet program integrity, or ensure compliance in case of audit.
- CDC-recognized organization collection of and submission to CDC of required program evaluation data elements for purposes of receiving “pending,” “preliminary,” and “full recognition” National DPP lifestyle change program designation status (for more information, see DPRP requirements).
- Medicaid MCO submission of program cost data to state Medicaid agency for purposes of refining health plan rates.
- Experience has shown that provider referrals and sharing National DPP lifestyle change program results with a participant’s primary care provider can increase program enrollment and retention.
Commercial Plans
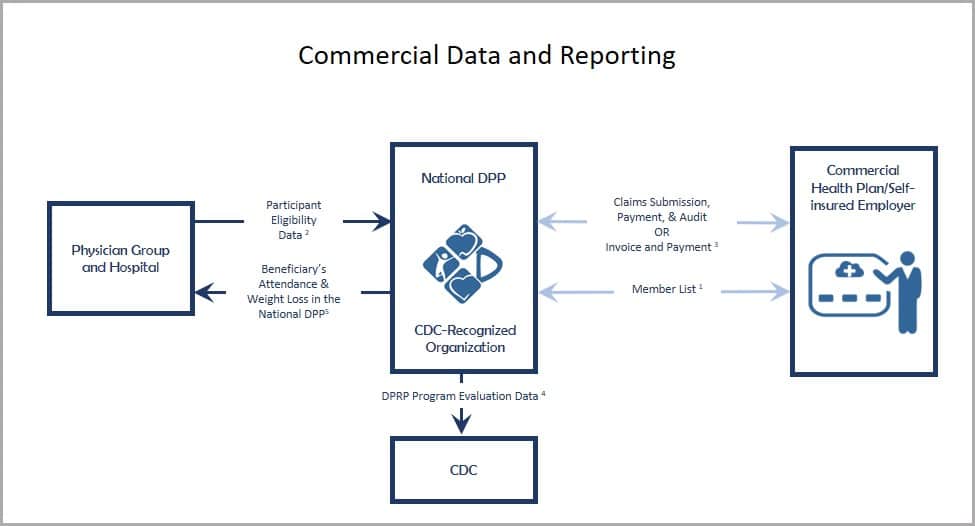
- Commercial plan submission of member lists to CDC-recognized organizations, generated based on an analysis of historical claims data, to identify enrollees for outreach efforts.
- Physician group and hospital submission of electronic health record (EHR) data to a commercial plan or CDC-recognized organization to identify enrollees for outreach efforts. (For more information, see Program Delivery: Screening & Identification)
- CDC-recognized organization submission of a claim or invoice and encounter data to the commercial plan, employer, or third party organization for reimbursement and evaluation purposes.
- CDC-recognized organization collection, and submission to CDC, of required program evaluation data elements for purposes of receiving pending, preliminary, and full recognition. (For more information, see DPRP requirements)
- Experience has shown that provider referrals and sharing National DPP lifestyle change program results with a participant’s primary care provider can increase program enrollment and retention.
Data Security and Regulatory Compliance
Payers will want to ensure that CDC-recognized organizations with which they work have the capacity to meet all statutory and regulatory requirements pertaining to privacy and data security. At a basic level, CDC-recognized organizations will need to be able to ensure the privacy and confidentiality of the data their program participants will be sharing with them.
Business Associate Agreement (BAA) or Data Use Agreement (DUA)
As noted above, CDC-recognized organizations will need to comply with HIPAA, the HITECH Act, and all applicable state privacy and data security statutes and relevant regulatory requirements issued by state insurance commissioners or other state regulatory authorities.
Payers will enter into agreements with the CDC-recognized organizations they contract with to ensure data security and regulatory compliance. A sample BAA developed by the American Medical Association (AMA) can be accessed here.
A BAA or DUA will likely be used and may include the following elements:
- Permitted and prohibited uses of Protected Health Information (PHI) and nonpublic personal financial information: outlines that uses of PHI and nonpublic personal financial information must comply with all applicable privacy and security laws, including HIPAA.
- Obligations for privacy and security breaches: outlines that a CDC-recognized organization may have obligations, such as reporting obligations, if there is a privacy or security breach. Examples of breaches include information systems being exposed to a virus or worm, an individual using company data through unauthorized access, an attack compromising a server, or unauthorized access or disclosure of PHI.
- Obligations upon termination: outlines obligations upon the conclusion of a BAA, including the return or destruction of PHI or continued protection of PHI.
- Required security controls: required security controls may include (a(n)):
- Information security program – such as written policies for security and the identity of the individual responsible for enforcement of the security program.
- Audit plan – may include who can complete an audit and how frequently the audit must be conducted.
- Approved encryption – required use of approved encryption for the transfer of confidential information to and from the Medicaid MCO and to and from third-parties.
- Network and systems security programs/tools – required use of security programs such as an industry standard malware detection program, an intrusion detection or prevention system, and firewalls that separate networks containing confidential information from public networks. Medicaid MCOs may also require third-party annual penetration testing of both internal and external systems.
- Data destruction agreement – outlines the type of data that must be destroyed, the circumstances under which destruction is required, and the method of destruction required.
- Physical and system controls – may include required use of endpoint protection for remote access of confidential information, keeping operating systems updated, safeguarding hard copies with a clean desk policy, and/or retaining visitor logs for the facility.
- Controls on workforce members accessing information – examples include background checks prior to providing employee access to confidential information, only providing access to employees who have a legitimate need to use the information as part of their job responsibilities, using IDs and passwords to access confidential information, and providing security awareness trainings prior to granting employees access to confidential information.
- Cloud storage controls – additional controls may be necessary if data is stored using a cloud-based technology.
- Business continuity and disaster recovery plan – plans outlining the critical information an organization needs to continue operating during an unplanned event or disaster needs be documented and tested regularly.
- Incident response plan – to be documented and tested regularly.
Data Sharing and Ownership
It is critical that all parties have a clear understanding around National DPP lifestyle change program data sharing and ownership needs and that all agreements pertaining to data sharing and ownership are reflected in any relevant contracts, business associate agreements, and memoranda of understanding. This is especially key for CDC-recognized organizations.
CDC-recognized organizations are required to share deidentified program data with the CDC’s DPRP to maintain recognition. They do not need a data-sharing agreement with the CDC to provide this information. They may also need to share data with their parent organization, a third-party program evaluator, or other entity on a case-by-case basis. For data to be shared with groups other than the CDC, data sharing agreements may be required.
Evaluation
Developing a comprehensive evaluation plan will support efforts to improve the National DPP lifestyle change program and can be done in any context, such as during a pilot, when the program is a fully covered Medicaid benefit, or within a commercial context. The data collected during an evaluation can be used for many purposes, including to guide benefit design, measure changes over time against a baseline, and establish accountability for the use of resources.
When developing an evaluation plan, it is important to identify the questions of interest, how needed information will be collected, and how the results will be shared and utilized. Answering these questions will help the evaluation team define roles and responsibilities as they relate to the evaluation and ultimately set the evaluation up for success.
Potential Evaluation Questions
When implementing an evaluation of the National DPP lifestyle change program, it is important to start by defining the questions to be answered. Potential evaluation questions include:
- How were the delivery models implemented and what factors may have influenced implementation?
- How many (and what proportion) of the state’s Medicaid population diagnosed with or at risk for prediabetes were engaged, enrolled, retained, and completed the National DPP lifestyle change program?
- How did delivery programs retain Medicaid participants? What were the factors associated with retention?
- Did Medicaid participants achieve the expected outcomes to meet the standards of the DPRP? Which participants were most likely to achieve these outcomes? What benefits did participants experience through participation in the program? What were the social/behavioral outcomes?
Measures for Consideration
In 2022-2023, grantees participating in the Medicaid Beneficiary Enrollment Project (MBEP)* focused on the following measures for evaluation related to advancing access to the National DPP lifestyle change program to Medicaid populations:
- Total adult Medicaid beneficiaries in the state
- Total Medicaid beneficiaries in the state eligible for the National DPP lifestyle change program
- Number of eligible referrals
- Referral sources
- Referral to enrollment conversion rates/source conversion
- Number of Medicaid beneficiaries enrolled
The following graphic provides examples of how the measures listed above can guide the selection or implementation of supporting measures within an evaluation of the National DPP lifestyle change program.
Hover over the green plus signs on the graphic below to see the supporting measures.
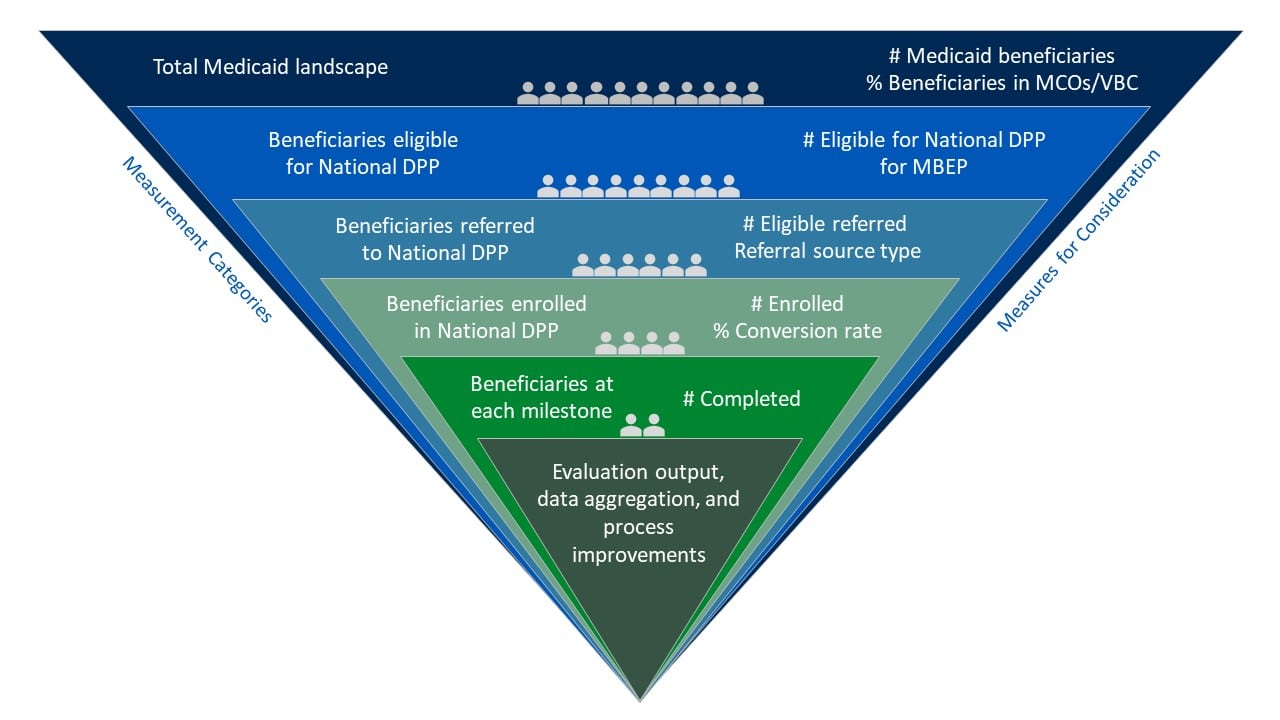
*MBEP was an opportunity for states to receive funding and group-based technical assistance from CDC Division of Diabetes Translation and NACDD to increase access to and enrollment in the National DPP lifestyle change program for Medicaid beneficiaries.
Data Considerations
Data Sources and Collection Methods
There are many ways to evaluate a program or pilot, and the best approach will be determined by your question(s) and the available data. The data sources described in the Types of Data section of this page could be utilized as key data sources when evaluating the National DPP lifestyle change program benefit or pilot. Additional data sources for evaluation could include primary data collected through surveys, interviews, or focus groups.
When determining appropriate data collection methods, consider the complexity associated with each method and the resources and staffing available to carry out the evaluation. Below are examples of data collection methods and how they may be used to evaluate the National DPP lifestyle change program.
| Data Collection Method | Potential Use |
| Pre/post participant survey |
|
| Clinical data monitoring |
|
| Survey of participating CDC-recognized organizations |
|
Timing
When planning an evaluation for the National DPP lifestyle change program, it is important to consider what indicators can reasonably be measured in a given timeframe. It can take time to demonstrate the impact of the National DPP lifestyle change program. For example, demonstrating return on investment (ROI) often takes at least three years. It can take up to six months alone for Medicaid claims to become available for evaluation purposes. As a result, it is important for stakeholders to understand the timeline of the evaluation from the beginning. It may also be beneficial to build in indicators or measurements that can be looked at periodically throughout the evaluation process, such as referrals to the program, enrollment in each session, or number of cohorts.
For more information about the ROI for the National DPP lifestyle change program, please see the Cost and Value page of the Coverage Toolkit.
Evaluation Examples
Medicaid Coverage for the National DPP Demonstration Project
Incorporating Multiple Evaluation Methods
During the Medicaid Coverage for the National DPP Demonstration Project, NACDD worked with Research Triangle Institute (RTI) International to evaluate the following:
- Process for Medicaid coverage and delivery of the National DPP lifestyle change program in Maryland and Oregon
- Cost of the different delivery models
- Enrollment, engagement, and retention strategies
- Participant outcomes for demonstration participants
The multimethod evaluation of the Medicaid Coverage for the National DPP Demonstration Project included:
- Program implementation surveys, cost surveys and interviews with states, MCOs in Maryland, coordinated care organizations (CCOs) in Oregon, and CDC-recognized organization staff
- Focus groups with Lifestyle Coaches
- Pre/post telephone surveys with Medicaid beneficiary participants
The executive summary of the evaluation report was released on 1/10/19. Learn more about the evaluation of the Medicaid Coverage for the National DPP Demonstration Project here.
Michigan
Utilizing Evaluation Results
As part of Michigan Department of Health and Human Services’ (MDHHS) MCO pilot of the National DPP lifestyle change program, MDHHS collected data to evaluate each CDC-recognized organization that received previously available 1815 funding through the pilot. MDHHS collects a variety of data including:
- Cost per participant to provide the program
- Enrollment in the program
- Attendance at sessions
- Weight loss across participants
These data were utilized to evaluate whether participating organizations were meeting MDHHS requirements, which were modeled after the current DPRP standards.
After obtaining needed data, MDHHS’ timeline for evaluation prompted them to give technical assistance to CDC-recognized organizations that were not meeting DPRP standards. If CDC-recognized organizations were not reaching DPRP standards halfway through the contract year, MDHHS worked with them to create a performance improvement plan. If at the end of the contract year the CDC-recognized organization had still not achieved the standards, pilot reimbursement was decreased incrementally. From MDHHS’ perspective, this process of utilizing evaluation data to drive technical assistance created a realistic model for pay-for-performance that buildt capacity for CDC-recognized organizations.
Michigan used data from the pilot to demonstrate why statewide Medicaid coverage of National DPP lifestyle change program should be prioritized by Michigan Medicaid, including cost learnings from the pilot and cost projections for state-wide coverage. Provider, MCO, and internal MDHHS champions helped to showcase the work accomplished during the pilot. This data has since led to state-wide Medicaid coverage of the Michigan Diabetes Prevention Program (MiDPP).
To learn more, see Michigan’s State Story of Medicaid Coverage on the Coverage Toolkit.
Montana
Evaluating Multiple Aspects of the National DPP Lifestyle Change Program
Montana is a state that has conducted rigorous evaluation on various aspects of the National DPP lifestyle change program since implementing Medicaid coverage. Montana’s evaluation studied the following:
- Program adaptations for the Medicaid population
- Effectiveness of the program for individuals with disabilities
- Effects of financial incentives for program participants
- Differences in program effectiveness based on age
- Effectiveness of telehealth delivery of the program to rural populations
To learn more, visit Montana’s State Story of Medicaid Coverage on the Coverage Toolkit.
State Data
Understanding the impact of prediabetes and type 2 diabetes in a state or in a community can lead to a greater understanding of the importance of prevention efforts like the National DPP lifestyle change program.
Examples of How States Present Data
- Utah Medicaid Coverage of the National DPP One-Pager
- Colorado DSMES Education Brief and Analysis
- Colorado National DPP One-Pager
- Virginia HB 1098 Potential Solution National DPP Brief
State Data Sources and Ideas
Using the data sources noted in the table below, states and their partners can curate specific data to help build the case for coverage of the National DPP lifestyle change program. More details on the data sources in the table and additional sources are provided in the section below. Please note these are hypothetical examples, and do not represent real data from specific states.
| Ideas for Using State Data to Make the Case for Coverage | Where to Gather These Data |
| Diabetes prevalence and costs: 11.3% of adults in XX state have type 2 diabetes. This equates to 565,000 adults statewide and results in an estimated $3.8 billion annual cost to XX state. Additionally, an estimated 1 in 3 adults have prediabetes, which equates to nearly 2 million adults in the state. Adults with prediabetes are at high risk of developing type 2 diabetes, which results in significant health and economic costs. | Data sources: State-specific BRFSS data and the CDC Diabetes Atlas provide diabetes prevalence estimates. The ADA provides estimates of costs. Data notes: Multiplying the total state population by the share of the population with diabetes and prediabetes can provide an estimate of the number of people living with these conditions. Providing both a percentage and a number can help a reader better understand the magnitude of the problem. Note that if diabetes prevalence data are for adults only, then only the adult population total should be used in calculations. Example calculation: 11.3% (adult diabetes prevalence) X 5,000,000 (adult population in state) = 565,000 adults with diabetes. |
Prevalence of type 2 diabetes and prediabetes in Medicaid:
Diabetes and prediabetes are more prevalent in the Medicaid population. For example:
|
Data sources: State Medicaid agencies can use Medicaid claims data to determine the number of Medicaid beneficiaries with diagnosed type 2 diabetes and the estimated number of beneficiaries who could be eligible for the National DPP lifestyle change program.
Data notes: States may use a variety of methods to estimate the number of individuals eligible for the program, including:
|
The cost of type 2 diabetes and prediabetes to Medicaid and possible cost savings of the National DPP lifestyle change program:
Diabetes can lead to serious health problems and result in significant health care costs. For example:
|
Data sources: State Medicaid agencies can calculate the average annual cost per enrollee for Medicaid beneficiaries with and without diabetes. Taking the difference between these two averages provides an estimate of how much excess money is spent on diabetes-related expenses. Example calculation: $13,000 (average annual cost per enrollee with diabetes) - $5,000 (average annual cost per enrollee without diabetes) = $8,000 (estimate of excess money spent on diabetes-related expenses). Data notes: Contextualizing the values obtained through the calculation above, with the number of Medicaid beneficiaries with prediabetes (see row above), and the estimated cost of the program, can further illuminate the benefits of the National DPP lifestyle change program and the potential for savings. Example contextualization: $8,000 (estimate of excess money spent on diabetes-related expenses per enrollee per year, see example calculation above) vs. $700-$1,500 (estimate of the cost of the National Diabetes Prevention lifestyle change program per enrollee per year). Many studies have been conducted on diabetes-related costs in Medicaid; several of these studies are summarized here. |
| The cost of type 2 diabetes medication compared to the cost of the National DPP lifestyle change program: The cost of medication used to treat type 2 diabetes is high, particularly medication classes GLP-1, SGLT2, DPP-4 and the newer combination GIP/GLP-1. The American Diabetes Association (ADA) recommends using these medication classes when co-morbid conditions (including high blood pressure, end-stage renal disease, and cardiovascular disease) exist rather than lower cost alternatives like metformin. These medication classes can be among the top 25 most expensive medications for a Medicaid managed care plan. Retail prices for these medications may range from $400 to $1500 per month, which is similar to the cost of providing the National DPP lifestyle change program for one year. | Data sources: Estimates for the amount of money spent on diabetes-related medication can be found using data from Good Rx, but these are retail prices, which are likely discounted for Medicaid. A state Medicaid office or a Medicaid managed care organization (MCO) can provide more exact cost estimates for diabetes-related medication. |
| Highlighting how the National DPP lifestyle change program can address health equity: Some areas in XX state face higher social vulnerability based on the CDC’s social vulnerability index. These areas also tend to be lower-income and have higher rates of diabetes. Offering the National DPP lifestyle change program through Medicaid can help reduce these disparities and improve health equity. | Data sources: The CDC Diabetes Atlas and Social Vulnerability Index maps social vulnerability, or the potential negative effects on communities caused by external stresses on human health. This tool provides data down to the county-level, which is a more regional view of risk. |









